科技工作者之家
科界APP是专注科技人才,知识分享与人才交流的服务平台。
科技工作者之家 2020-03-06
来源:研之成理
▲第一作者:佟月宇;通讯作者:梁骥,周思,刘健 通讯单位: 天津大学/伍伦贡大学(梁骥)、大连理工大学/伍伦贡大学(周思)、大连化学物理研究所/大连-萨里未来材料研究中心(刘健)论文DOI:10.1002/anie.202002029
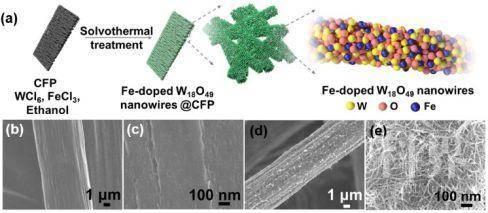 ▲Figure 1. (a) Schematic illustration of the preparation of Fe-doped W18O49 nanowires @ CFP. SEM images of (b-c) CFP, (d-e) W18O19-16Fe nanowires @ CFP.
▲Figure 1. (a) Schematic illustration of the preparation of Fe-doped W18O49 nanowires @ CFP. SEM images of (b-c) CFP, (d-e) W18O19-16Fe nanowires @ CFP.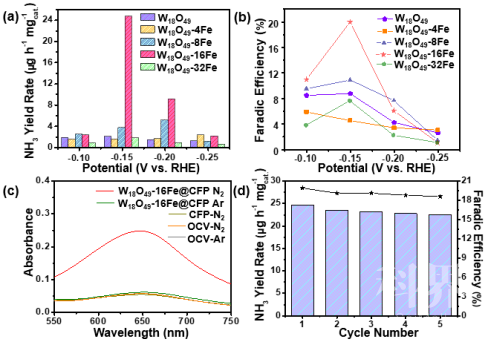 ▲Figure 2. (a) NH3 yield rate at various potentials vs. RHE. (b) Faradic efficiency of as-prepared Fe-doped W18O49 nanowires @ CFP electrodes. (c) UV-vis absorption spectra of various electrolytes stained with salicylic acid indicator. OCV stands for open-circuit voltage. (d) cycling stability results at −0.15 V (vs. RHE) of W18O49-16Fe nanowires @ CFP electrode.
▲Figure 2. (a) NH3 yield rate at various potentials vs. RHE. (b) Faradic efficiency of as-prepared Fe-doped W18O49 nanowires @ CFP electrodes. (c) UV-vis absorption spectra of various electrolytes stained with salicylic acid indicator. OCV stands for open-circuit voltage. (d) cycling stability results at −0.15 V (vs. RHE) of W18O49-16Fe nanowires @ CFP electrode.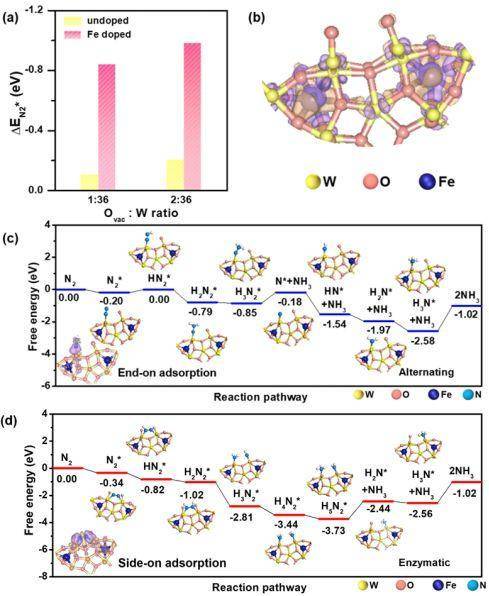 ▲Figure 3. (a) N2 adsorption energy (ΔEN2*) as a function of Ovac : W ratio for W18O49 with and without Fe doping by DFT calculations. (b) The differential charge density between Fe and W18O49 with Ovac : W ratio of 2 : 36 without N2 chemisorption. Purple and orange colors represent electron accumulation and depletion regions, respectively, with isosurface value of 5×10−3 eÅ−3. (c, d) Free energy diagrams of NRR on Fe-doped W18O49 with Ovac : W ratio of 1 : 36 and 2 : 36, respectively. The structures of reaction intermediates are shown next to their energy segments. Left bottom of (c, d) inserted the differential charge density distributions of N2 chemisorbed on Fe-doped W18O49 with Ovac : W ratio of 1 : 36 and 2 : 36, respectively. The Fe/W ratio is fixed at 11%.
▲Figure 3. (a) N2 adsorption energy (ΔEN2*) as a function of Ovac : W ratio for W18O49 with and without Fe doping by DFT calculations. (b) The differential charge density between Fe and W18O49 with Ovac : W ratio of 2 : 36 without N2 chemisorption. Purple and orange colors represent electron accumulation and depletion regions, respectively, with isosurface value of 5×10−3 eÅ−3. (c, d) Free energy diagrams of NRR on Fe-doped W18O49 with Ovac : W ratio of 1 : 36 and 2 : 36, respectively. The structures of reaction intermediates are shown next to their energy segments. Left bottom of (c, d) inserted the differential charge density distributions of N2 chemisorbed on Fe-doped W18O49 with Ovac : W ratio of 1 : 36 and 2 : 36, respectively. The Fe/W ratio is fixed at 11%.来源:rationalscience 研之成理
原文链接:https://mp.weixin.qq.com/s?__biz=MzIwMzE5MzQ1NQ==&mid=2649343924&idx=4&sn=4a659f9dc532b6e4e0e656d72eea4e69&chksm=8ece4ab4b9b9c3a2d45e18c56ef8b7c24b80617bb2481b157f49a29e76cfd9885f617cecc0a0#rd
版权声明:除非特别注明,本站所载内容来源于互联网、微信公众号等公开渠道,不代表本站观点,仅供参考、交流、公益传播之目的。转载的稿件版权归原作者或机构所有,如有侵权,请联系删除。
电话:(010)86409582
邮箱:kejie@scimall.org.cn

今日科技话题:4000米深海自持式剖面浮标海试成功、家庭社会地位影响儿童多动症发病率、首次实现陶瓷4D打印、解析金刚鹦鹉起源
南开大学教授周其林院士:以化学“催化”育人之美




研究揭示纳米反应器在液相加氢反应中的空间限域效应

科研人员研发出单原子修饰的纳米反应器
大连化物所研发出单原子修饰的纳米反应器

马大为:对有机化学的探索永无止境 | 2018年未来科学大奖获奖人

单位点金属催化剂的微环境调控及纳米反应器工程在电催化二氧化碳还原反应中的进展与展望

纳米反应器让胰腺癌检测“快准稳”

专访周其林:无比享受探索未知的“催化”大师

用纳米反应器对胰腺癌进行诊断