科技工作者之家
科技工作者之家APP是专注科技人才,知识分享与人才交流的服务平台。
科技工作者之家 2020-09-08
来源:宏基因组
TM:宿主-细菌界面的MicroRNAs:宿主防御或细菌攻击
MicroRNAs at the Host–Bacteria Interface: Host Defense or Bacterial Offense
Review, 2018-11-23
MicroRNAs at the Host-Bacteria Interface: Host Defense or Bacterial Offense
Trends in Microbiology, [11.776]
10.1016/j.tim.2018.10.011
日报MicroRNA(miRNA或微RNA)是一类小的非编码RNA,是基因表达的转录后调节因子;
微RNA的变化可以反映宿主对感染的反应,也可以是细菌病原体为破坏宿主的关键功能而设计的策略;
微RNA对宿主细胞周期的扰动,可能通过维持其增殖生态位而促进细菌的生存和/或增殖;
多个细菌病原体的感染导致了微RNA表达的改变,从而调节了自噬的多个步骤,进而抑制细菌降解。
摘要MicroRNAs是一类小的非编码RNA,是基因表达的主要转录后调节因子。它们在宿主和细菌病原体之间复杂的相互作用中发挥着重要作用,既可以作为宿主免疫反应的一部分来中和感染,也可以作为细菌为了自身利益而劫持宿主途径的分子策略。在这里,我们总结了近年来微生物在哺乳动物宿主被细菌病原体感染过程中的作用,强调了关键的细胞途径。此外,我们还讨论了这一领域的新兴主题,包括小RNA参与宿主-微生物群串扰和细胞间通讯。
MicroRNAs are a class of small noncoding RNAs that act as major post-transcriptional regulators of gene expression. They are currently recognized for their important role in the intricate interaction between host and bacterial pathogens, either as part of the host immune response to neutralize infection, or as a molecular strategy employed by bacteria to hijack host pathways for their own benefit. Here, we summarize recent advances on the function of miRNAs during infection of mammalian hosts by bacterial pathogens, highlighting key cellular pathways. In addition, we discuss emerging themes in this field, including the participation of miRNAs in host-microbiota crosstalk and cell-to-cell communication.
图1. MiRNAs在感染过程中起着多方面的作用,控制着多个宿主细胞的功能。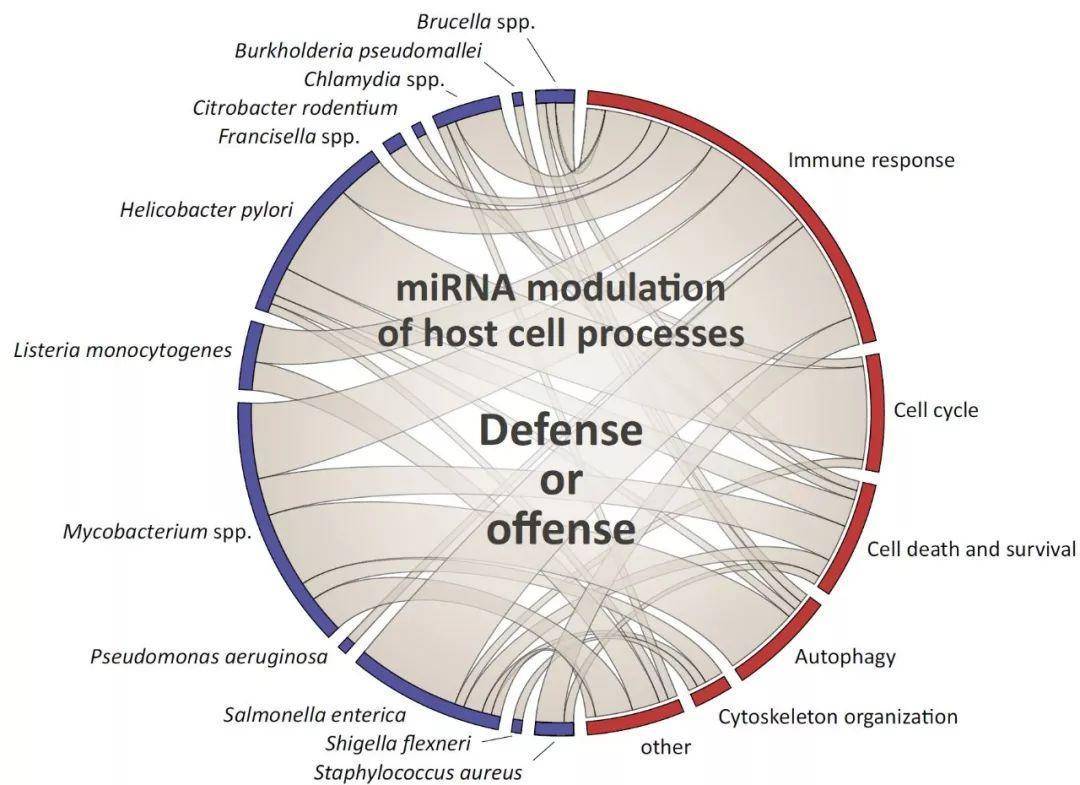
小RNA的变化可以反映宿主对感染的反应,也可以是细菌病原体为破坏宿主的关键功能而设计的策略。条带宽度大致表示每个关系所描述的小RNA数目。
Figure 1. MiRNAs Play Multifaceted Roles during Infection, Controlling Multiple Host Cell Functions. MiRNA changes can reflect host responses to fight infection or can be a strategy devised by bacterial pathogens to subvert critical host functions. Ribbon width represents roughly the number of miRNAs described for each relationship.
图2. 微RNA对细菌性病原体感染的调节导致宿主细胞周期的改变。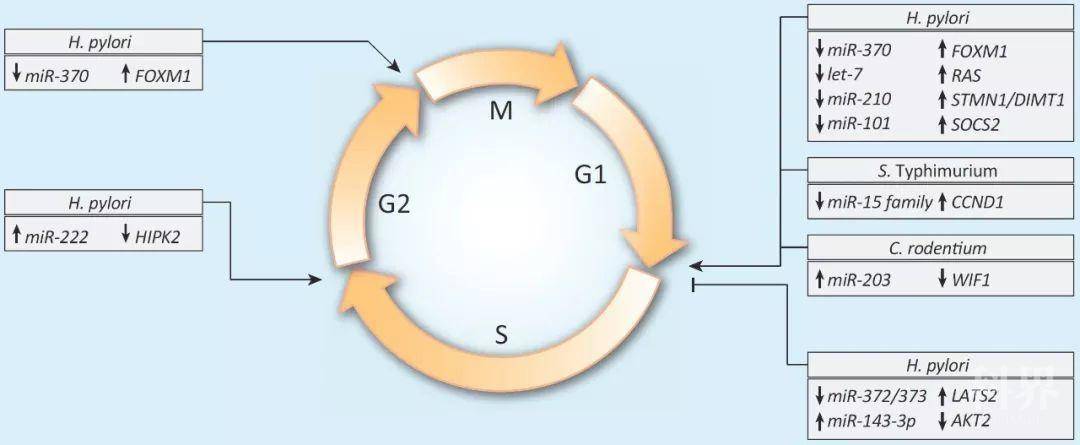
宿主细胞周期的扰动可能通过维持其增殖生态位而促进细菌的生存和/或增殖。幽门螺杆菌;鼠伤寒杆菌;肠沙门氏菌;鼠伤寒沙门氏菌;鼠柠檬酸杆菌。
Figure 2. MiRNA Modulation upon Infection by Bacterial Pathogens Induces Alterations of the Host Cell Cycle. Perturbations of the host cell cycle might contribute to bacterial survival and/or proliferation by maintaining their proliferative niche. H. pylori, Helicobacter pylori; S. Typhimurium, Salmonella enterica serovar Typhimurium; C. rodentium, Citrobacter rodentium.
图3. 细菌感染后的微RNA变化对自噬有很大影响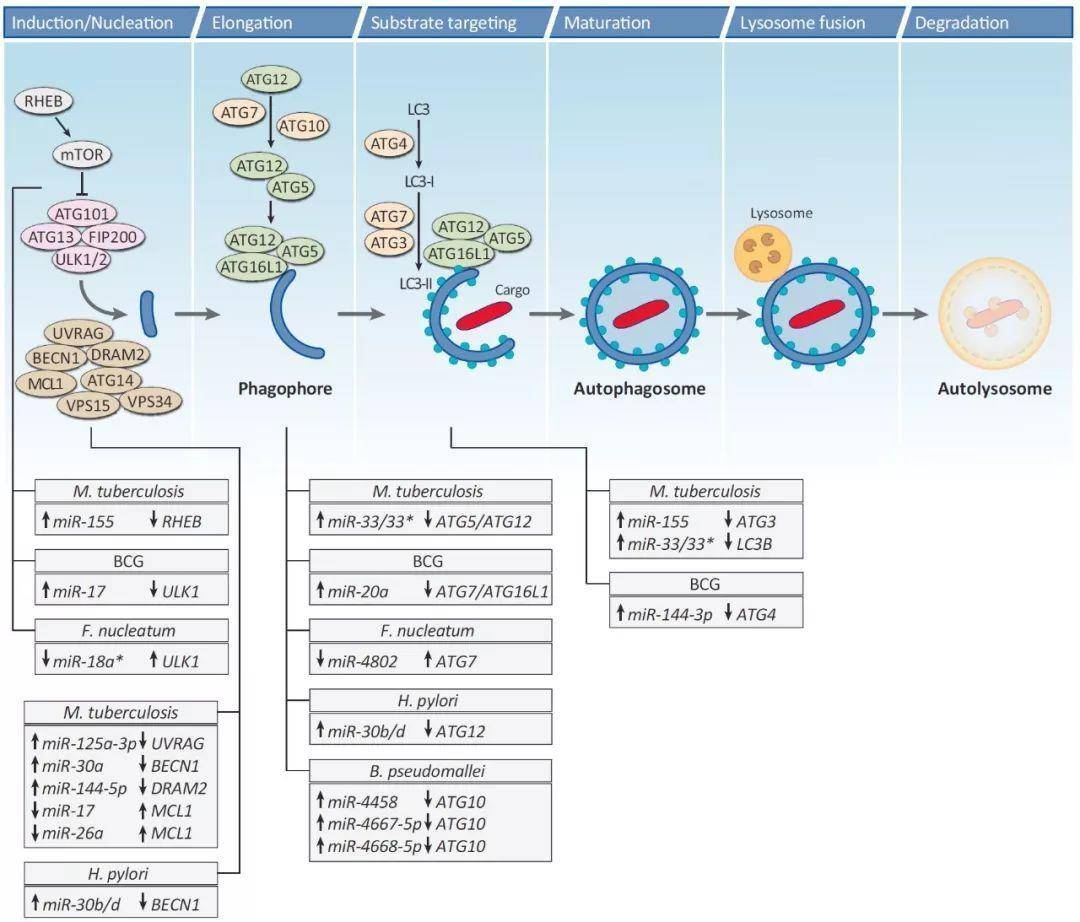
多个细菌病原体的感染导致了微RNA表达的改变,从而调节了自噬的多个步骤。感染过程中微RNA调节的一个反复出现的主题是抑制自噬,从而抑制细菌降解。结核分枝杆菌,结核分枝杆菌;有核梭杆菌;幽门螺杆菌;假锤骨杆菌,假锤骨伯克霍尔德菌;卡介苗。
Figure 3. MiRNome Changes upon Bacterial Infection Have a Strong Impact on Autophagy. Infection by multiple bacterial pathogens leads to alterations of miRNA expression, which regulate multiple steps of autophagy. A recurring theme underlying miRNA modulation during infection is the inhibition of autophagy and, hence, of bacterial degradation. M. tuberculosis, Mycobacterium tuberculosis; F. nucleatum, Fusobacterium nucleatum; H. pylori, Helicobacter pylori; B. pseudomallei, Burkholderia pseudomallei; BCG, Bacillus Calmette–Guérin.
来源:meta-genome 宏基因组
原文链接:https://mp.weixin.qq.com/s?__biz=MzUzMjA4Njc1MA==&mid=2247492013&idx=5&sn=0405d68d60c01f8bfc06b6df42d1f050&chksm=faba0b1ccdcd820afa71786ec5c25b34946f3d4d495a0cef32c23a038e3e50ce0ca9559a11cf#rd
版权声明:除非特别注明,本站所载内容来源于互联网、微信公众号等公开渠道,不代表本站观点,仅供参考、交流、公益传播之目的。转载的稿件版权归原作者或机构所有,如有侵权,请联系删除。
电话:(010)86409582
邮箱:kejie@scimall.org.cn

病毒和细菌如何在肠道微生物群中相互平衡

哈佛大学研究显示:结肠癌细胞会在转移时携带细菌
瘤胃微生物
微生物所等揭示细菌III型分泌系统调控机制
微生物所揭示植物识别病原细菌的新机制
银与细菌“携手”,微生物燃料电池效率倍增

你牙菌斑上的微生物,可能和土壤细菌是亲戚
叶面微生物
微生物测量
“第七届植物病原细菌学术研讨会”在南京召开 12月9日,由中国植物病理学会


