科技工作者之家
科技工作者之家APP是专注科技人才,知识分享与人才交流的服务平台。
科技工作者之家 2019-09-25
来源:逻辑神经科学
Authorship︱Zdenek Lansky and Marcus Braun, Richard McKenney and Kassandra Ori-McKenney et al. Nat. Cell Biol.
Source︱Gwyneth Zakaib, Alzforum
Editor︱Sizhen Wang, LT-Neuroscience
Two papers in the September 2 Nature Cell Biology (the least IF 17.728) present complementary evidence that tau condenses into a patchwork of rafts that coat microtubules. These patches share some properties with phase-separated liquid droplets of tau that have been described recently. Zdenek Lansky and Marcus Braun of the Czech Academy of Sciences, Prague West, and Amayra Hernández-Vega at the Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany, led one of the studies, while husband-and-wife team Richard McKenney and Kassandra Ori-McKenney of the University of California, Davis, led the other. Both groups report that these islands of tau regulate movement of microtubule-associated motors and fend off enzymes that would cut up the tubulin strands. Together, the papers suggest that tau condensation plays a major role in its physiological function, and that it is key to microtubule regulation[1-2].


· Tau condenses in monolayer patches on microtubules.
· Patches regulate movement of molecular motors and action of enzymes.
· Loss of healthy tau condensation could lead to microtubule degradation.
“These studies open up a new way of thinking about tau, and recognize it as a liquid, condensed phase in the physiological context,” said Susanne Wegmann, DZNE, Berlin.
Benjamin Wolozin, Boston University School of Medicine, agreed. “These studies extend our understanding of the importance of phase separation in biology, and might ultimately impact on our understanding of the pathophysiology of tauopathies,” he wrote to Alzforum.
Tau is a microtubule-associated protein (MAP) known to stabilize microtubules, regulate traffic of other MAPs, and protect against tubulin proteolysis[2-4]. It was thought to bind microtubules as individual molecules, although some reports suggested tau self-associates along the microtubule and forms intermittent solid patches[5-6]. Two years ago, reports began to surface that tau undergoes liquid-liquid phase separation—condensing into droplets in solution. Does this play a role in normal, healthy function and could it be important for microtubule biology?
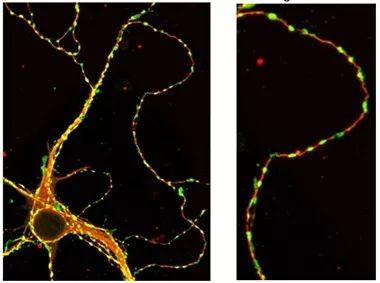
Strung Along. On a hippocampal neuron from a mouse (left), patches of tau (green) line up on along microtubules (red). Magnification at right[2].
To find out, both groups of researchers used total internal reflection fluorescence (TIRF) microscopy to watch how tau behaved on microtubules assembled in the lab. In this technique, a laser focuses on one tiny area of the specimen at a time, minimizing background fluorescence and improving spatial resolution down to the single-molecule level.
Through this technique, both groups saw that tau bound the microtubule surface in two ways, either diffusely as individual molecules or in dynamic islands of condensed tau proteins that dotted the length of microtubules.The islands—cohesive, single-layer coatings of tau—were of uniform density and grew and shrank from their outer boundaries. Islands fused when they met along the microtubule length. Their formation required a minimum concentration of soluble tau.
Within these islands, tau’s mobility slowed relative to tau bound to microtubules as single molecules. Island tau exchanged more slowly with free tau and took longer to detach from microtubules once free tau was removed from solution. The findings suggested that within these condensed patches, tau molecules interact with one another.
Working with Braun, co-first authors Valerie Siahaan and Jochen Krattenmacher at the Czech Academy of Sciences found that while the kinesin-1 motor could easily move between islands, it fell off microtubules as soon as it encountered an island. On the other hand, the molecular motor Kip3 moved through islands, albeit at a slower velocity than it traveled between them. Condensed tau blocked microtubule digestion by the hydrolase katanin.
Writing in the McKenney paper, first author Ruensern Tan reported that the islands allowed movement of dynein complexes, but were impervious to the microtubule-severing enzyme spastin. Together, the data suggest that tau islands form selectively permissible barriers that spatially regulate the location and action of other microtubule-associated proteins.
As reported in the McKenney paper, the tau-tau interactions on microtubules are similar to, though distinct from, those occurring when tau condenses in the liquid phase. Both condensates are reversible, depend on tau concentration, can fuse with neighboring droplets/islands, and can be dissolved by the alcohol 1,6 hexanediol. However, the single-layer tau condensation on microtubules does not take on a gel-like consistency, nor does it exchange as quickly with tau in solution. This suggests that the microtubules have a stabilizing effect on tau, say the authors.
The findings have raised many questions. Wegmann wondered how physiological and pathological phosphorylation affects island dynamics. Braun is examining if tau mutations might do the same. Hernández-Vega speculates that other microtubule proteins may form similar patches that spatially sort proteins and regulate transport.
TIRF microscopy is a powerful technique, commented Khalid Iqbal, New York State Institute for Basic Research, Staten Island, New York. He cautioned that it remains to be seen whether tau islands exist in vivo. Though the authors found tau patches on neurons in mice (see image above), all the mechanistic experiments were done in vitro.
“One of the biggest take-home messages of our work is that the self-interaction of tau molecules is not just a disease-specific phenomenon, but rather may be a common molecular process for tau to perform its normal cellular functions,” wrote the McKenneys to Alzforum. “The loss of normal condensate dynamics could be detrimental to cellular control over these processes.”
That could have implications for health. Since a minimum concentration of soluble tau is required for islands to form, neurofibrillary tangles or other aggregates that sequester the protein could limit island formation. “Microtubules would then fall apart because they would be eaten up by enzymes such as katanin,” said Braun.
“In addition to the important cell biological insights reported in these two papers, they imply the necessity for caution as the field moves toward trials for AD and other tauopathies intended to reduce total tau,” wrote Kenneth Kosik, University of California, Santa Barbara. “Therapeutic strategies that reduce total tau synthesis, such as the use of antisense oligonucleotides, will affect pools of tau on microtubules and consequently microtubule function in ways that are not easily predictable.”
来源:LT-Neuroscience 逻辑神经科学
原文链接:https://mp.weixin.qq.com/s?__biz=MzI4Mjk3NzUxOQ==&mid=2247485163&idx=2&sn=cedf397a741cbd8df6912353d679385c&chksm=eb90f36bdce77a7d299b01df68897d536c39572b792b740e33d50942381fa8cd03bb7b2c46ef&scene=27#wechat_redirect
版权声明:除非特别注明,本站所载内容来源于互联网、微信公众号等公开渠道,不代表本站观点,仅供参考、交流、公益传播之目的。转载的稿件版权归原作者或机构所有,如有侵权,请联系删除。
电话:(010)86409582
邮箱:kejie@scimall.org.cn

活动预告丨“CAAI进校园”系列活动走进电子科技大学
Cell :翻译后修饰介导tau蛋白致病性的分子结构多样性
提供治疗Tau蛋白相关神经退行性疾病的新治疗靶标——低密度脂蛋白相关蛋白1

JAMA Neurol:Aβ、tau蛋白与认知衰退
tau蛋白上的又一个新修饰——琥珀酰化修饰

EMBO Rep | 刘恭平、王建枝教授团队发现Tau蛋白聚集神经损伤的机制
皮克病tau蛋白纤维结构破解
浙大34岁“歌神教授”:科研也是在创造艺术品



科学家绘制tau蛋白细节图

研究进展:Nature-错误折叠Tau 蛋白的结构菌株定义了不同的疾病